
At time $t=0$ the water vapor pressure $e$ is zero, and the vacuum above the water surface begins to "fill" with water vapor molecules that "escape" the water surface because they have sufficient kinetic energy to overcome the intermolecular bonding of the fluid phase. The escaping water molecules come from the region close to the water surface. The rate of evaporation is given by $R_e$, and it is a function of temperature almost entirely. The greater the temperature, the greater the rate of evaporation.

As time increases, the mass of water vapor increases in the region above the water surface. Now, there is sufficient vapor pressure $e$ to create a condensation rate $R_c$, although less than the rate of evaporation $R_e$.
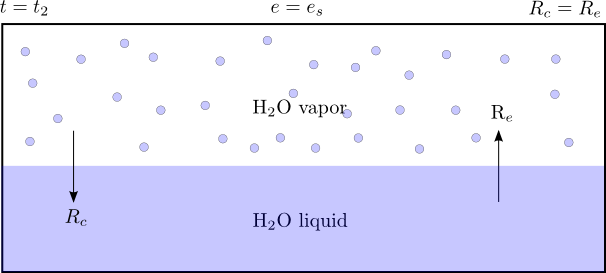
The vapor pressure $e$ will increase as long as $R_e > R_c$. Eventually, the vapor pressure will reach the saturation value $e_s$. When this occurs, the rates of evaporation and condensation are equivalent, and the vapor pressure will no longer increase. Because the rates of evaporation and condensation are equal, some scientists believe the phrase "equilibrium vapor pressure" more aptly describes this state than "saturation vapor pressure."


