

|

|
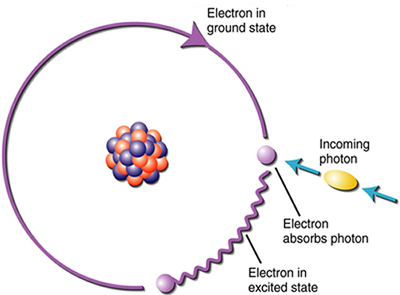
The particle-like behavior of electromagnetic energy is observed in its interaction with atoms or molecules. The energy level of a photon, or packet of light, depends on the wavelength of the light wave. The shorter the wavelength, the higher the energy. Electrons that orbit the nucleus of the atom have only a finite number of discrete energy levels. The electron will only move to its next higher energy level when it absorbs a photon with energy exactly equal to the difference in energy of the two adjacent orbits. The atom absorbs the photon and the electron moves to the next higher state. When the electron eventually emits the absorbed energy, it does so in the form of a photon with the same energy as the photon that was initially absorbed.